|
Description:
|
|
Cara Jacobson, Kristin Lasseter, MD, David Puder, MD There are no conflicts of interest for this episode. Dr. Kristin Yeung Lasseter is a renowned reproductive psychiatrist who has dedicated her career to the intersection of mental health and reproductive medicine.
As the founder of Reproductive Psychiatry and Counseling, Dr. Lasseter has been instrumental in expanding access to reproductive psychiatry services in Texas but also worldwide through her teaching and online presence. Through her steadfast devotion to comprehending the singular hurdles faced by individuals as they navigate the reproductive journey, she has garnered immense respect within the field.
Dr. Kristin Yeung Lasseter's profound contributions to advancing women's mental health in Central Texas have been recognized through the prestigious Association of Women Psychiatrists Symonds Fellowship in 2018. Through her expertise, compassion, and advocacy, she is transforming lives and dismantling the stigma associated with perinatal mental health.
Of note, this episode, and the article below is for information purposes only and we recommend talking with a specialist doctor when considering what is the risk and benefits of particular medications in an individual's specific situation. Risks Of Untreated Peripartum Mental IllnessUntreated and active mental illness adversely affects both mother and fetus; therefore, it is vital to appropriately assess and treat perinatal mental illness for better outcomes for both. There is a myth that pregnancy is protective against psychiatric illness, but mental health conditions, including suicide and overdose/poisoning related to substance use disorder, account for a larger share of all US pregnancy-related deaths (23%) than any other cause (Centers for Disease Control [CDC], 2022). Untreated depression in pregnancy is one of the strongest risk factors for postpartum depression, which can have devastating consequences like suicide and infanticide (Payne, 2021). More pregnant women die from suicide than hemorrhage or preeclampsia (Viswanathan et al., 2021). Perinatal mental illness is associated with many additional adverse outcomes, including preterm delivery, pre-eclampsia, gestational diabetes, small-for-gestational-age (SGA), fetal distress, neonatal hypoglycemia, adverse neurodevelopmental outcomes, impaired maternal-infant bonding, disordered attachment, infantile colic, and increased use of harsh discipline (Betcher & Wisner, 2020; Payne, 2021). Psychiatric illness is also associated with high-risk maternal behaviors during pregnancy, like indiscriminate sex and exposure to sexually transmitted infections, substance use, less prenatal care, and poor nutrition (Betcher & Wisner, 2020). Systematic review and meta-analysis of 191 eligible studies with a combined sample of 195,751 unique mother-child dyads found that offspring of mothers with perinatal depression or anxiety had impaired social-emotional, cognitive, language, motor, and adaptive behavior development, extending beyond infancy and into adolescence (Rogers et al., 2020). Children exposed to peripartum depression have higher cortisol levels, a finding that lasts into adolescence; antenatal depression treatment may result in more normal cortisol levels in infants (Payne, 2021).

See also:
Psychiatric Medication In PregnancyUnfortunately, overall quality of evidence on the risks of psychiatric medications in pregnancy is low and confounded by the effects of the underlying conditions. This is illustrated by a 2021 systematic review of psychopharmacotherapy in pregnant, postpartum, or reproductive‐age women in which 95% of the included studies reporting on adverse effects were observational and could not fully account for confounding variables (Viswanathan et al., 2021).
However, it is clear that there are significant harms associated with untreated perinatal mental illness and we know that discontinuing psychiatric medication in pregnancy is associated with relapse of mental illness; in particular, there is a 60-70% risk of relapse in pregnant women with a history of major depressive disorder who discontinue antidepressants and an 80-100% risk of recurrence in women with bipolar disorder not taking medication compared to 29-37% chance in women continuing treatment with mood stabilizers while pregnant (Payne, 2021). Thus, it is important to appropriately screen for and treat perinatal psychiatric illness.
Deciding whether or not to start or continue perinatal psychiatric medications requires ‘risk-risk’ analysis. In general, both patients and clinicians tend to overestimate medication risks in the peripartum period but underestimate the risks associated with untreated psychiatric illness (Horan et al., 2022). Certainly, all patients do not require medication, and psychotherapy, for example, is an effective treatment in appropriately selected patients, but for those with features like moderate-severe symptoms, suicidality, or functional impairment (e.g., inability to care for self or baby), medication is likely indicated.
According to Payne (2021): When possible, it is ideal to make changes 6-12 months prior to attempting pregnancy to ensure mood stability. Minimize the number of medications if possible, considering the patient’s history and potential negative effects of psychiatric illness on the child.
Most psychiatric medications can be continued in pregnancy. Bottom line: if the patient is psychiatrically ill, treat the illness.
When caring for patients with unplanned pregnancy (Payne, 2021): See the patient as soon as possible. Do not stop all psychiatric medications immediately. Consider stopping teratogenic medications. If discontinuing a medication, taper when possible. If switching medications, remember baby will be exposed to at least 2 drugs, in addition to the risk of potential relapse.
Psychiatric Medications In LactationAlthough the benefits of breastfeeding on both maternal health and child development in general have been clearly established, there is little definitive evidence on the safety of most medications in nursing mothers, impacts on infant development, or the effects on lactation itself (Jordan et al., 2022). Research on pharmacotherapy and lactation is limited due to various factors, including lack of standardization in population databases, bidirectional effects of breastfeeding and medication use, prenatal medication exposure, and the direct impacts of breastfeeding (or not) on infant health and development, which “may obscure the true relationship between medicine exposure during pregnancy and developmental outcomes” (Jordan et al., 2022). The beneficial effects of breastfeeding may, in fact, offset some of the potential harms of medication exposure through pregnancy or lactation.
For infants already exposed in utero, Payne (2021) proposes that it may be unnecessary to switch while breastfeeding unless: The patient experiences relapse or the current regimen is not working Risk of severe side effects with continued exposure (e.g., clozapine) Infant experiences medical complications or side effects from the medication during breastfeeding Sedation is the most common side effect. Monitor for sleepiness or poor feeding, especially when breastfeeding after a maternal medication dose. If applicable, monitor infant blood levels. While uncommon, antipsychotics can cause stiffness, cogwheeling, and EPS in the baby.
Psychotropic medications pass into breast milk to varying degrees. For example, sertraline is secreted in low amounts, but lithium secretion is relatively high: For exclusively breastfeeding mothers taking sertraline, the estimated infant dose is 0.5% of the maternal weight-adjusted dose (Drugs and Lactation Database (LactMed®) [LactMed], 2022c). A case series of 11 breastfeeding mothers found that the infant dose of lithium ranged from 0 to 30% of the maternal weight-adjusted dose, with an average of 12.2% (LactMed, 2022m).
Further details are discussed later in this article, but as a broad overview: Most antidepressants can be used in lactation, and generally, breastfeeding mothers needing antidepressant medication should take one that has previously been effective for them (Anderson, 2021). For breastfeeding patients requiring mood stabilizers, lamotrigine can be used (LactMed, 2022e). Valproic acid also appears to be safe in lactation (LactMed, 2022k), but other agents are preferred in women that can become pregnant given its teratogenicity. Lithium can be used in lactation, as well, particularly in healthy, full-term infants (especially those over 2 months old), although this may require close monitoring of infant behavior and/or blood levels (LactMed, 2022m). Some anxiolytics can decrease breastmilk supply or cause sedation in breastfed infants, requiring close monitoring (LactMed 2021a, 2021b, 2022g, 2023b, 2023c). There is less research on antipsychotics, but second generation antipsychotics seem to be relatively safe in lactation, with the exception of clozapine, which has been shown to cause adverse effects in breastfed infants (Uguz, 2016). Some antipsychotics may also increase or decrease breastmilk supply due to dopamine blockade (LactMed, 2022f, 2022i, 2022j, 2022l, 2023e). Stimulant medications for ADHD, in particular methylphenidate, can be used in nursing mothers who require treatment (LactMed, 2023d). Methadone and buprenorphine can be used in lactation for opioid use disorder (Nagpal et al., 2020).
The American Academy of Pediatrics (AAP) recommends against parents sharing a sleeping surface with infants (bed sharing) due to the risks of sudden infant death syndrome (SIDS), strangulation, suffocation, and entrapment and notes that the baseline risk increases by more than 10 times when someone in the bed has impaired alertness or is less able to arouse due to fatigue, substance use, or sedating medications, including certain antidepressants and other psychiatric medications (Moon et al., 2022). Bed sharing is sometimes referred to as co-sleeping, although this term is imprecise and can also refer to room sharing, where parents sleep in the same room as the infant but on separate sleep surfaces, which is recommended by the AAP and may decrease the risk of SIDS by up to 50%. Of note, they also report that breastfeeding is associated with decreased risk of SIDS. Further resource: LactMed®Drugs and Lactation Database (LactMed®) is a fully-referenced database that provides information about levels of drugs and other substances in breastmilk and infant blood, possible adverse effects on nursing infants, and appropriate therapeutic alternatives. AntidepressantsDeligiannidis & Freeman (2014) report that women are about twice as likely as men to have major depressive disorder (MDD), and approximately 1 in 5 women experience perinatal depression. When left untreated, antepartum depression increases the risk of obstetrical and neonatal complications, and postnatal depression has been found to significantly negatively impact child development (Deligiannidis & Freeman, 2014).
Numerous observational studies have examined the neurodevelopmental impacts of prenatal antidepressant exposure, and several have suggested adverse effects on children’s language, cognition, and academic performance. However, these deficits are generally mitigated or eliminated when controlling for maternal depression and other confounding factors (Andrade, 2022).
A recent study of standardized exam performance in children aged 9-15 found no change in language scores and a small but significant decrease in math scores (~2 points out of 100) associated with gestational exposure to antidepressants (Andrade, 2022). These findings persisted but were attenuated when accounting for various confounders. It is likely that the remaining deficits after adjustment in this and other studies result from “residual confounding from unmeasured behavioral and internal environment variables associated with untreated maternal depression” (Andrade, 2022). Overall, it appears that prenatal antidepressant exposure may in fact be only a marker of maternal depression and not a direct cause of neurodevelopmental deficits, which seem to result primarily from the consequences of underlying mental illness, rather than medication effects.
While the existing literature does not support withholding antidepressants in perinatal depression, it remains important to use shared decision-making in conversations about medication use (Andrade, 2022). Selective Serotonin Reuptake Inhibitors (SSRIs)SSRIs have been studied the most and are the most commonly used antidepressants in pregnancy or lactation, including: fluoxetine (Prozac), citalopram (Celexa), escitalopram (Lexapro), and sertraline (Zoloft). Neonatal Adaptation SyndromeAll SSRIs carry increased risk of neonatal adaptation syndrome (NAS), a self-limited, frequently mild condition presenting similarly to withdrawal, typically presenting with symptoms like irritability, jitteriness, respiratory distress, seizures, and hypoglycemia (Muzik & Hamilton, 2016). Several studies have linked exposure to SSRIs in late pregnancy with transient neonatal distress syndromes thought to be caused by withdrawal from antidepressants and possibly affecting ~25% of babies with prenatal SSRI exposure close to the time of delivery (Massachusetts General Hospital [MGH] Center for Women’s Mental Health, 2022). Symptoms include: tremors, restlessness, increased muscle tone and increased crying, but these syndromes appear to be benign and resolve quickly–within 1-4 days–without medical intervention (MGH Center for Women’s Mental Health, 2022). Limitations include that most studies did not blind the mother’s treatment status, possibly leading to more precautionary admissions to special care nursery in infants with known medication exposure but no serious clinical manifestations, and maternal mood was not assessed, resulting in confounding, as untreated antenatal depression and anxiety are known to contribute to poor neonatal outcomes, including premature delivery and low birth weight (MGH Center for Women’s Mental Health, 2022).
Persistent Pulmonary HypertensionPersistent pulmonary hypertension (PPH) is a serious condition affecting newborns in which high blood pressure in the pulmonary arteries leads to inadequate oxygenation of the blood. There are reports of increased PPH with SSRI exposure, but results were not clinically significant, and the risk appears to be lower than originally thought (Muzik & Hamilton, 2016). Some studies have also found an association between SSRI use after 20 weeks gestation and increased risk of persistent pulmonary hypertension of the newborn (PPHN), but the estimated risk is small, affecting less than 1% of infants exposed to SSRIs, and subsequent studies have not found a link between antenatal antidepressant use and PPHN (MGH Center for Women’s Mental Health, 2022). Still, some clinicians advise women to taper or discontinue SSRIs in late pregnancy, but there is not good evidence that this improves neonatal outcomes, which are affected by both treated and untreated mental illness. Importantly, decreasing or discontinuing antidepressants prior to delivery may increase the risk of postpartum depression (MGH Center for Women’s Mental Health, 2022).
Postpartum HemorrhageThere is some evidence that SSRIs can interfere with platelet function, resulting in bleeding, and there does appear to be an association between SSRIs and SNRIs and postpartum hemorrhage, although the quality of evidence is low, and further research is needed (Viswanathan et al., 2021).
Congenital Malformations and DevelopmentNeither SSRIs nor SNRIs are linked to increased risk of birth defects or changes in mental development after adjusting for confounders associated with underlying mental illness (Betcher & Wisner, 2020). Prenatal exposure to tricyclic antidepressants or fluoxetine has not been shown to impact IQ, language, temperament, mood, or behavioral development in preschoolers (MGH Center for Women’s Mental Health, 2022; Nulman et al., 1997). When controlling for underlying psychiatric illness, gestational exposure to SSRIs does not appear to increase the risk of autism spectrum disorders (Kobayashi et al., 2016).
Heart DefectsSome studies have demonstrated a link between antenatal SSRI exposure and heart defects, but most did not control for underlying mental illness, and subsequent studies with more appropriate control groups have failed to show the same association, including a study with a sample size over 900,000 (Payne, 2021). Several meta-analyses have examined associations between antidepressants (SSRIs, sertraline, citalopram, fluoxetine, paroxetine, and TCAs) and cardiac anomalies but used varying inclusion criteria “that did not consistently exclude cardiac anomalies associated with prematurity” and odds ratios ranged from 0.86 to 1.26, with wide confidence intervals crossing the null (Viswanathan et al., 2021).
LactationBreastfeeding mothers who need antidepressant medication should generally take one that has worked well for them in the past (Anderson, 2021). Some consider sertraline and paroxetine the drugs of choice for breastfeeding women that have not previously used antidepressants due to low levels in breast milk, largely undetectable infant serum levels, and few infant adverse effects (Anderson, 2021). Fluoxetine has a longer half life of 4-6 days and an active metabolite (norfluoxetine) with half life around 7-15 days, which can result in drug accumulation in breastfed infants and infant serum levels up to 59% of maternal levels that decline slowly after stopping breastfeeding or the mother discontinues the medication (Anderson, 2021). If a mother used fluoxetine during pregnancy, most experts do not recommend switching to a different medication for breastfeeding (Anderson, 2021). Citalopram and escitalopram have relative infant doses and half-lives in between those of fluoxetine and sertraline or paroxetine; they can also be used in lactation, particularly if the patient is currently stable on either medication or it has been previously helpful (Anderson, 2021).
Avoidance of Paroxetine (Paxil) in PregnancyThis is controversial, but generally, it seems that exposure to paroxetine is safer than exposure to untreated mental illness (Lasseter, 2022). If a patient is stable on paroxetine, it is likely reasonable to continue it during pregnancy, especially if there is a history of severe mental illness, high risk of relapse, or other medications have been ineffective. Some studies have shown increased risk of heart defects, but others have not. The risk of cardiac defects with paroxetine is lower than once thought and has not been borne out in studies controlling for confounders like maternal depression severity (Huybrechts et al., 2014). As with other antidepressants, there is a higher risk of neonatal adaptation syndrome, but this is usually mild and of short duration (Payne, 2021). Similar to other SSRIs, paroxetine is only found in small amounts in breastmilk, and no significant adverse effects have been reported due to exposure to paroxetine through breastfeeding (LactMed, 2022b).
Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)Congenital MalformationsProspective data shows exposure to venlafaxine (Effexor) during the first trimester does not increase risk of major malformations (MGH Center for Women’s Mental Health, 2022). There is insufficient data about duloxetine (Cymbalta) (MGH Center for Women’s Mental Health, 2022). As with SSRIs, prenatal exposure to SNRIs is not associated with increased risk of birth defects or changes in mental development after adjusting for confounders associated with underlying mental illness (Betcher & Wisner, 2020).
LactationAlthough research is limited, there have not been reported adverse effects on breastfed infants with duloxetine, and breastmilk levels are very low with a relative infant dose under 1% (Anderson, 2021). Venlafaxine has an active metabolite (desvenlafaxine) with a longer half life, and the relative infant dose for both substances is ~6.5%, with breastfed infant serum levels ranging from undetectable to 37% of maternal levels; there have been reports of drowsiness or agitation in exposed infants, but most do well, and venlafaxine can be used with caution in lactation (Anderson, 2021).
Bupropion (Wellbutrin)Congenital MalformationsBupropion use in pregnancy has not been associated with major malformations (Payne, 2021). Older reports showed increased rates of heart and great vessel malformation with infants exposed to bupropion, but a retrospective cohort study of 1200 infants with bupropion exposure did not show increased risk of cardiovascular malformations, and the Bupropion Pregnancy Registry data shows rates of congenital malformation (3.9%) similar to those with no known teratogen exposure (MGH Center for Women’s Mental Health, 2022).
LactationBupropion can be used with caution in breastfeeding patients, but there is limited data on its use in lactation, and there have been case reports of possible seizures in breastfed infants, so other medications may be preferred, especially with newborns or premature infants (LactMed, 2023a).
Tricyclic Antidepressants (TCAs)Of the TCAs, desipramine and nortriptyline are preferred in pregnancy as they are less anticholinergic and may reduce the risk of orthostatic hypotension, which is common in pregnancy (MGH Center for Women’s Mental Health, 2022).
Congenital MalformationsLactationAmitriptyline has been associated with extreme drowsiness in a breastfed infant, although other infants have tolerated exposure to this medication well (Anderson, 2021). Nortriptyline is an active metabolite of amitriptyline but seems to have fewer adverse effects and does not have any active metabolites of its own, so some consider nortriptyline a drug of choice (along with sertraline and paroxetine) in breastfeeding women that have not previously tried antidepressants (Anderson, 2021). Doxepin should be avoided in breastfeeding due to reports of serious infant adverse effects (Anderson, 2021).
Monoamine Oxidase Inhibitors (MAOIs)PregnancyMAOIs are usually avoided during pregnancy, as they can lead to hypertensive crises if combined with tocolytic medications like terbutaline, and their use in pregnancy has not been well studied (MGH Center for Women’s Mental Health, 2022).
LactationOther AntidepressantsIn a prospective study, none of nefazodone, trazodone, and mirtazapine increased rates of congenital malformation (MGH Center for Women’s Mental Health, 2022).
Brexanolone (Zulresso)AllopregnanoloneAllopregnanolone is a naturally occurring neuroactive progesterone metabolite and GABA-A receptor modulator; when used as a medication, the synthetic analogue of allopregnanolone is called brexanolone (Standeven et al., 2022). Standeven et al. (2022) report that women with more peripartum mood and anxiety symptoms had lower levels of allopregnanolone in the second trimester (although this was not statistically significant) and higher levels of allopregnanolone at 6 weeks postpartum; these trends persisted whether patients had a psychiatric history or not.
Use in Perinatal DepressionAn intravenous preparation of brexanolone (brand name Zulresso) was approved in March 2019 by the FDA and is the first drug specifically designed for postpartum depression (Mughal et al., 2022). It is only recommended for those with severe postpartum depression whose symptoms are refractory or who decline treatment with antidepressants or ECT (Mughal et al., 2022). Brexanolone has limited availability and requires continuous monitoring for adverse effects like sedation, loss of consciousness, and hypoxia during a 60 hour infusion (Mughal et al., 2022). The long-term safety and efficacy still need to be examined (Mughal et al., 2022).
EfficacyBased on 3 RCTs on brexanolone in a total of 209 women with postpartum depression, the least square mean Hamilton Rating Scale for Depression (HAM-D) scores improved 4.1 points more than placebo at 60 hours and 2.6 points over placebo at 30 days, both of which were statistically significant (Viswanathan et al., 2021). Given that the Hamilton Rating Scale for Depression (HAM-D) total scores range from 0 to 50, the clinical significance of a 4.1 point improvement can depend on the individual judgment of the treating physician to weigh this potential improvement in the context of other factors, including potential side effects, cost-effectiveness, and feasibility of the treatment regimen.

Table adapted from MDCalc (n.d.) based on the Hamilton Rating Scale for Depression (1960)
AnxiolyticsBenzodiazepinesDue to limited data, the neurodevelopmental effects of prenatal exposure to benzodiazepines remain uncertain (Wang et al., 2022). Based on the findings of 2 meta-analyses, 2 registry-based studies, and 2 large retrospective cohort studies, pre-pregnancy or prenatal use of benzodiazepines and z-hypnotics is associated with multiple adverse outcomes (Andrade, 2023): The meta-analyses reported increased rates of spontaneous abortion, induced abortion, preterm birth, low birth weight, small for gestational age, low 5-minute Apgar scores, and neonatal intensive care unit (NICU) admission associated with benzodiazepine and/or z-hypnotic use in pregnancy (Grigoriadis et al., 2020, 2022). One of the large cohort studies found an increased risk of ectopic pregnancy with benzodiazepine use in the 90 days prior to conception (Wall-Wieler et al., 2020). The other large cohort study by Noh et al. (2022) found a small but statistically significant increase in risk of overall congenital malformations and cardiac malformations in a South Korean nationwide sample. In contrast, the two registry studies (Lee et al., 2022; Spuznar et al., 2022) and 2022 meta-analysis by Grigoriadis et al., as well as a previous meta-analysis (Grigoriadis et al., 2019), did not find an increased risk of congenital malformations.
However, it is unclear to what degree these associations result directly from medication exposure, as opposed to possible confounding by indication, confounding by severity of indication, or residual confounding (Andrade, 2023).
LactationMedications with antihistamine properties like hydroxyzine and diphenhydramine could decrease breastmilk supply, especially with prolonged use, larger doses, in combination with sympathomimetics like pseudoephedrine, or before breastfeeding is well-established (LactMed, 2021a, 2021b). Benzodiazepines can be used in lactation, but infants should be monitored for sedation and problems with feeding or growth (LactMed, 2022g, 2023b, 2023c). Lorazepam has a shorter half-life than many benzodiazepines thus is preferred over other drugs with longer half-lives, like alprazolam and clonazepam (LactMed, 2022g, 2023b, 2023c).
Mood Stabilizers and Anti-epileptic drugs (AEDs)There are more studies looking at these medications used for epilepsy in pregnancy, not directly investigating psychiatric indications like bipolar disorder. Payne (2021) recommends encouraging all pregnant women taking anticonvulsants to take high-dose folate (4 mg per day), which can theoretically decrease the risk of neural tube defects. Valproic acid and carbamazepine should be avoided in pregnancy but can be used in lactation (Payne, 2021).
Avoid Valproic Acid, Valproate, and Divalproex (Depakote) in Women of Childbearing PotentialThe most important psychiatric medications to avoid in pregnancy are valproic acid and its derivatives: Valproate is the conjugate base of valproic acid (National Center for Biotechnology Information, 2023b). Divalproex is a combination of valproic acid and valproate (National Center for Biotechnology Information, 2023a).
In general, do not prescribe these medications to women of childbearing potential, even if not planning pregnancy in the near future. However, if a nursing mother requires valproic acid treatment, breastfeeding does appear to be safe (LactMed, 2022k).
Congenital MalformationsA 2021 review by Kaplan and Demir reported estimates of major congenital malformations associated with prenatal valproate/valproic acid exposure ranging from 6.2% to 17.4%, and rates of major congenital malformations were ~2-5 times higher compared to control groups. The risk of neural tube defects (particularly spina bifida) is approximately 1-2%, which is 10-20 times higher than in the general population (Kaplan & Demir, 2021). With a neural tube defect risk around 1-6%, valproic acid should only be used as a last resort in women of child-bearing potential due to the high risk of teratogenicity early in pregnancy, often before a woman may be aware of the pregnancy (MGH Center for Women’s Mental Health, 2022).
Effects on Development, Autism, and Intellectual DisabilityThe effects of prenatal exposure to valproic acid seem to be dose-dependent and are apparent as early as infancy, with lower developmental quotient (DQ) compared to controls (Bromley & Baker, 2017). Lower intelligence quotient (IQ) in school children, impaired language functioning, memory, social skills, and motor development have also been reported, in addition to increased risk of neurodevelopmental disorders (Bromley & Baker, 2017). A population-based cohort study with nearly 4.5 million participants looked at rates of autism and intellectual disability in children born to mothers with epilepsy and found that in those who were not exposed to AEDs, 1.5% were diagnosed with autism spectrum disorder (ASD), and 0.8% were diagnosed with intellectual disability by age 8 years (Bjørk et al., 2022). In same-aged children of mothers with epilepsy exposed to valproate monotherapy, 2.7% had a diagnosis of ASD, and 2.4% were diagnosed with intellectual disability (Bjørk et al., 2022). 4.3% of those exposed to topiramate had ASD, and 3.1% had intellectual disability (Bjørk et al., 2022).
There was no consistently elevated risk of neurodevelopmental disorders associated with prenatal exposure to monotherapy with lamotrigine, carbamazepine, oxcarbazepine, gabapentin, pregabalin, clonazepam, levetiracetam, or phenobarbital (Bjørk et al., 2022). Data from Bjørk et al. (2022):
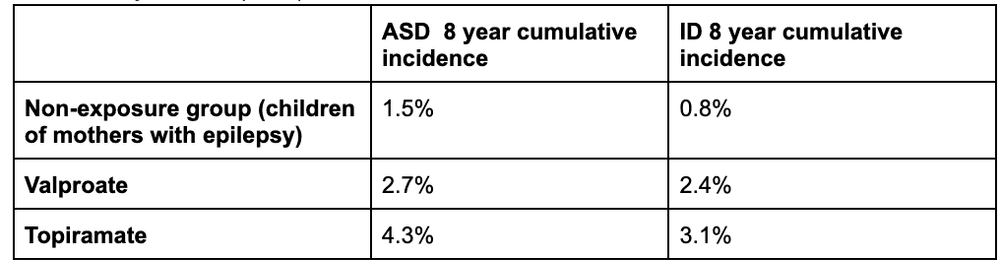
ASD: autism spectrum disorders; ID: intellectual disability
LactationAlthough it is contraindicated in pregnancy, valproic acid can be used in lactation, and there do not seem to be adverse effects on growth or development in breastfed infants (LactMed, 2022k). There is very little data specifically on divalproex, although it is rapidly metabolized to valproic acid (LactMed, 2022h). In fact, one study on children of mothers with epilepsy treated with anti-epileptic drugs (AED) in pregnancy found lower IQ at age 6 associated with prenatal valproate exposure but no adverse effects on IQ with breastmilk exposure to the studied drugs, valproate, carbamazepine, lamotrigine, and phenytoin (Meador et al., 2014). In children exposed to the studied AEDs, the adjusted mean IQ was 4 points higher in those who were breastfed than those who did not; for children exposed to valproate, adjusted mean IQ was 12 points higher in the breastfed group than the non-breastfed group (Meador et al., 2014). Valproic acid is excreted at low levels in breastmilk, and infant blood levels are low to undetectable (LactMed, 2022k). There is a theoretical risk of hepatotoxicity in breastfed infants, so they should be monitored for signs of liver injury like jaundice, although there have been no reported cases (LactMed, 2022k).
Avoid Carbamazepine (Tegretol) in PregnancyCarbamazepine use in pregnancy is associated with increased risk of congenital malformations, including neural tube defects, craniofacial abnormalities, skeletal disorders, hypospadias, and diaphragmatic hernia; it may also increase the risk of hemorrhage in the newborn (Payne, 2021). However, carbamazepine can be used in breastfeeding (LactMed, 2022d; Payne, 2021).
Lamotrigine (Lamictal)PregnancyThere is no significantly increased risk of major congenital malformations in babies with prenatal lamotrigine exposure (Kaplan & Demir, 2021). Analyses of a North American pregnancy registry indicated an increased risk of oral clefts in babies exposed to lamotrigine prenatally, but other registries failed to demonstrate similar findings (Kaplan & Demir, 2021). When used for seizures, the dose is often increased in pregnancy based on blood levels, but there is not clear evidence that blood levels are correlated with risk of relapse when used for bipolar disorder (MGH Center for Women’s Mental Health, 2020). It is best to check a lamotrigine level prior to pregnancy in a stable patient so you have a reference point for where to aim in pregnancy if their symptoms relapse (MGH Center for Women’s Mental Health, 2020). Recheck lamotrigine blood levels within the first 1-2 weeks postpartum, as they can increase after delivery, and the dosage may need to be adjusted, especially if it was increased significantly in pregnancy (MGH Center for Women’s Mental Health, 2020).
LactationThere do not appear to be adverse effects from lamotrigine monotherapy on growth or development in breastfed infants (LactMed, 2022e). Toxicity in breastfed infants is rare, but babies should be monitored for apnea, sedation, and poor sucking, as well as rash (LactMed, 2022e) Transient rashes have been reported in breastfeeding infants exposed to lamotrigine, and there is a theoretical risk of Stevens Johnson Syndrome (SJS), but there have been no reported cases of SJS (Khan et al., 2016). Infant serum levels should be checked if there is concern for toxicity, and some recommend checking the infant’s serum lamotrigine level, as well as platelets and liver function tests if the maternal dose is increased (LactMed, 2022e).
LithiumBlood LevelsLithium has a narrow therapeutic index, and it is important to closely monitor blood levels throughout pregnancy and delivery (Khan et al., 2016). Lithium dose often needs to be increased during pregnancy due to increased renal clearance and expanded maternal fluid volume, and it should be decreased after delivery as renal clearance and fluid volume begin to normalize postpartum (Khan et al., 2016). In order to minimize the serum concentration in infants, some recommend stopping lithium at the beginning of labor or 24-48 hours prior to scheduled Cesarean delivery, then restarting the pre-pregnancy dose after delivery (LactMed, 2022m). However, Dr. Lasseter generally recommends against doing so because postpartum psychosis can develop within days after birth, and lithium protects against this. Bergink and Kushner (2014) also advise that discontinuing lithium before delivery is unnecessary as long as maternal blood levels are in the therapeutic range.
Ebstein’s AnomalyPrenatal lithium exposure, particularly in the first trimester, has been associated with heart defects, but the risk is lower than initially reported; more recent studies estimate the absolute risk of Ebstein’s anomaly to be between 1 in 2000 and 1 in 1000 (0.05-0.1%) (MGH Center for Women’s Mental Health, 2022). Although there seems to be a small increase in cardiac malformations with lithium, it may be reasonable to start or continue it in pregnancy, especially in women with severe bipolar disorder, in whom the risk of relapse may outweigh the risk of Ebstein’s anomaly (Payne, 2021).
LactationSome recommend avoiding lithium in lactation due to high levels of excretion into breast milk. However, according to many sources, lithium use is not an absolute contraindication to breastfeeding, particularly with lithium monotherapy and in healthy, full-term infants, especially those older than 2 months (LactMed, 2022m). When using lithium in breastfeeding patients, the baby should be monitored for signs of toxicity like feeding difficulties, sedation, restlessness, or poor growth; some groups recommend monitoring infant blood levels at different intervals, while other groups recommend checking lithium levels only when there is a clinical suspicion for toxicity (LactMed, 2022m).
AntipsychoticsAlthough there is limited data, most antipsychotics appear to be relatively safe in pregnancy, with the exception of risperidone, which is associated with a small increase in congenital malformations (Payne, 2021). Additionally, postpartum psychosis, in particular, can have devastating consequences, including infanticide, so the benefits of antipsychotic treatment likely outweigh medication risks in patients with serious mental illness (Payne, 2021).
Olanzapine (Zyprexa)Olanzapine is associated with increased risk of gestational diabetes (Betcher & Wisner, 2020). Preliminary data from the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications show no major congenital malformations associated with first trimester olanzapine exposure in a small sample of 49 infants (Viguera et al., 2023). There have been reports of sedation in breastfed infants exposed to olanzapine, but overall, it is considered a first-line agent among second-generation antipsychotics in lactation (LactMed, 2022i).
Quetiapine (Seroquel)Based on four controlled studies, the pooled risk ratio for major malformations in infants with prenatal quetiapine exposure is estimated to be 1.03 (95% CI=0.89, 1.19), suggesting no increased risk (Cohen et al., 2018). Quetiapine is associated with increased risk of gestational diabetes (Betcher & Wisner, 2020). Breastmilk levels are low (<1% of the maternal weight-adjusted dose), and based on systematic reviews, quetiapine seems to be the first or second choice among second-generation antipsychotics in lactation (LactMed, 2022j).
Aripiprazole (Abilify)Among 163 infants exposed to aripiprazole in the first trimester, the risk of major malformations was not different compared to controls after adjusting for confounding variables (Freeman et al., 2021). Data on lactation is limited, and other medications with more data may be preferred over aripiprazole, especially if breastfeeding a newborn or premature infant (LactMed, 2022l). There have been reports of gynecomastia, galactorrhea, and cessation of lactation, in addition to impaired growth and weight loss in breastfed infants (LactMed, 2022l).
Risperidone (Risperdal)Risperidone (and potentially paliperidone) seems to increase the risk of overall and cardiac malformations (Betcher & Wisner, 2020). Risperidone may be considered as a second-line antipsychotic and used cautiously in lactation, but other agents are preferred due to higher breastmilk levels than other medications and reports of “Sedation, failure to thrive, jitteriness, tremors and abnormal muscle movements” in breastfed infants (LactMed, 2023e).
Clozapine (Clozaril)Clozapine does not appear to be teratogenic, although data is limited (Beex-Oosterhuis et al., 2021). Clozapine use in pregnancy is associated with 2x higher rates of gestational diabetes and may increase the risk of floppy infant syndrome and neonatal seizures (Mehta & Van Lieshout, 2017). Several sources recommend against breastfeeding when taking clozapine due to risks of sedation and agranulocytosis in the infant (LactMed, 2022a).
LactationData on antipsychotics and lactation is limited, but a 2016 systematic review of 37 reports found that second generation antipsychotics appear to be relatively safe in lactation, with the exception of clozapine (Uguz, 2016). Antipsychotics can cause hyperprolactinemia and resulting galactorrhea (milk production in men or nonpregnant, nonlactating women) due to dopamine blockade in the tuberoinfundibular pathway; this has been reported in several different medications, including haloperidol and risperidone (LactMed, 2022f, 2023e). Galactorrhea has also been reported in olanzapine, quetiapine, and aripiprazole, although these medications have a minimal effect on serum prolactin levels (LactMed, 2022i, 2022j, 2022l). In nursing mothers with established lactation, the effects of these antipsychotics on prolactin levels may not interfere with breastfeeding (LactMed, 2022f, 2022i, 2022j, 2022l, 2023e). There have also been case reports of aripiprazole associated with decreased breast milk supply (LactMed, 2022l).
Substance Use DisordersAn estimated 1-5% of pregnancies worldwide are affected by substance use disorders (Nagpal et al., 2020). A 2013 national survey reported that in the United States, 15.4% of pregnancies were exposed to cigarettes, 9.4% to alcohol, and 5.4% to illicit substances (Louw, 2018). Substance abuse causes many adverse effects in pregnancy and beyond, including fetal toxicity or teratogenicity, and overdose, which can sadly result in death and other devastating consequences. Pregnancy motivates many women to stop using, decrease use, or seek out substance use treatment, but some are unable to quit during pregnancy due to the chronic, relapsing nature of these disorders (Louw, 2018). Unfortunately, postpartum relapse rates are high, particularly in the first 6 months; one prospective study on substance use in pregnant women found that 83% were able to achieve abstinence from at least one substance, but 80% relapsed with at least one substance postpartum (Louw, 2018). Although criminalization does not lead to better outcomes for either mother or child, some states mandate reporting of all substance use in pregnancy to Child Protective Services (CPS), including prescriptions and medication-assisted treatment, and it is considered child abuse in 18 US states (Prince et al., 2023). Prenatal substance use is also grounds for involuntary commitment in 3 states (Prince et al., 2023). Many women avoid prenatal care or hide substance use from their care team due to fear of consequences like losing custody or being arrested (Prince et al., 2023). It is vital that we support our patients and adequately treat their substance use disorders before, during, and after pregnancy, practicing harm reduction when applicable.
Harm ReductionSee the Pregnancy and Substance Use: A Harm Reduction Toolkit from the CDC AlcoholFetal Alcohol Spectrum DisordersAlcohol is a well-known teratogen and can cause fetal alcohol spectrum disorders, which include a variety of adverse effects on physical, neurological, and behavioral development (Chung et al., 2021). In the United States, fetal alcohol syndrome is the most common cause of preventable intellectual disability (Prince et al., 2023).
Naltrexone and AcamprosateWhile there is little data on the safety of alcohol use disorder medications in pregnancy, acamprosate and naltrexone have not been associated with significant risks of congenital malformation or other serious adverse effects and should be considered in pregnancy given the known risks of alcohol consumption (Kelty et al., 2021).
DisulfiramOpioidsNeonatal Opioid Withdrawal Syndrome (NOWS)Prenatal opioid exposure can lead to neonatal opioid withdrawal syndrome (NOWS), which is associated with neurological, gastrointestinal, and respiratory adverse effects and may require prolonged hospitalization (Prince et al., 2023).
Methadone and BuprenorphineStimulantsPrescribed Stimulants vs Stimulants of AbuseIt is important to make the distinction between prescribed stimulant use (e.g., for ADHD treatment) versus substance abuse, which generally involves much higher doses and can have more severe effects on offspring. For example, prenatal methamphetamine use is associated with fetal growth restriction and low birth weight (Sankaran et al., 2022), as well as widespread effects on brain development and impairments in intellectual functioning, problem solving, short-term memory, and language development (Kunkler et al., 2022).
Attention-Deficit/Hyperactivity Disorder (ADHD)In patients with moderate to severe functional impairment, the benefits can outweigh risks of stimulant medication, such as improving safety while driving (Baker & Freeman, 2018).
MethylphenidateMethylphenidate has been the best studied, and most studies on stimulants used for ADHD in pregnancy have not found an association with malformations (Baker & Freeman, 2018). While some studies have found associations with prenatal exposure to stimulants and outcomes like miscarriage, low birth weight, placental problems, and low Apgar scores, others have found no change in risk for congenital malformations, prematurity, or birth weight (Baker & Freeman, 2018). It is possible that some of the adverse outcomes associated with these medications may be related to the underlying mental illness, rather than the medication alone (Baker & Freeman, 2018). Mothers who require methylphenidate can continue breastfeeding (LactMed, 2023d).
LactationMethylphenidate is undetectable in the serum of breastfed infants, and breastmilk levels appear to be very low, around 0.16%–0.7% of the maternal weight-adjusted dose (LactMed, 2023d). In typically-prescribed doses, dextroamphetamine does not appear to have adverse effects on nursing infants, and the breastfed infant dosage also seems to be relatively low: 5.7% of maternal dose in one study (Baker & Freeman, 2018).
TobaccoBehavioral InterventionsCounseling (e.g., motivational interviewing or cognitive behavioral therapy) and financial incentives are associated with decreased rates of smoking in pregnancy and lower risk of low birth weight (American College of Obstetricians and Gynecologists’ [ACOG] Committee on Obstetric Practice et al., 2020).
Bupropion (Wellbutrin; Zyban)Although data is limited, bupropion use in pregnancy is not associated with known risks of fetal anomalies, low birth weight, or preterm birth (ACOG Committee on Obstetric Practice et al., 2020).
Bupropion can be used with caution in breastfeeding patients, but there is limited data on its use in lactation, and there have been case reports of possible seizures in breastfed infants, so other medications may be preferred, especially with newborns or premature infants (LactMed, 2023a).
Varenicline (Chantix)Data on varenicline are very limited, but some small studies have not found evidence of teratogenicity (ACOG Committee on Obstetric Practice et al., 2020). Varenicline is not recommended in breastfeeding due to a lack of evidence, whereas the safety of bupropion in lactation has been somewhat better established (ACOG Committee on Obstetric Practice et al., 2020).
Nicotine Replacement TherapyWhile some reviews support the efficacy of nicotine replacement for smoking cessation in pregnancy, the evidence is inconsistent, and several US trials have been stopped early due to adverse effects or ineffectiveness (ACOG Committee on Obstetric Practice et al., 2020). Nicotine replacement therapy should only be considered in pregnancy with close monitoring and after detailed discussion of the known risks of continuing to smoke and the possible risks of nicotine replacement (ACOG Committee on Obstetric Practice et al., 2020). The authors of the LactMed® chapter on nicotine advise avoiding all forms of nicotine in nursing mothers (LactMed, 2020). The equivalent amount of nicotine from 17 cigarettes passes into the milk daily in breastfeeding mothers using a 21 mg transdermal nicotine patch (LactMed, 2020). Some advocate for smoking mothers using nicotine replacement in order to reduce breastfed infant exposure to smoke and toxins from cigarettes; however, animal data suggests that nicotine could interfere with infant lung development and may increase the risk of sudden infant death syndrome (SIDS) (LactMed, 2020).
Marijuana And Cannabinoids (CBD, THC, Delta-8, etc.)The use of marijuana and cannabinoids in the perinatal period seems to be increasing in the setting of more widespread legalization and availability (Martin, 2020). Cannabinoids have been shown to affect pregnancy through activating the endocannabinoid system, including CB1 and CB2 receptors (Martin, 2020). Perinatal cannabinoid use is associated with impairments in offspring behavioral, cognitive, and emotional development (Martin, 2020). Although there is no definitive data, marijuana has been associated with preterm birth, low birth weight, and stillbirth (Prince et al., 2023). The ACOG recommends avoiding marijuana in pregnancy, and the AAP recommends that mothers using marijuana should not breastfeed (Martin, 2020).
Perinatal Psychotherapy DepressionMost research on perinatal psychotherapy has studied patients with major depression or depressive symptoms and indicates that psychotherapy is a safe and effective treatment for perinatal depression. Psychological interventions such as therapy should be considered first-line treatment for perinatal depression (Cuijpers & Karyotaki, 2021). Cuijpers and Karyotaki (2021) found that psychological interventions were effective in treating perinatal depression, which remained significant at 12 months after treatment initiation, with an effect size of g=0.67 and number needed to treat (NNT) around 4. Although therapy may be less effective in patients with chronic depression, psychological interventions are effective, particularly in subthreshold depression where they may prevent progression to major depression (Cuijpers & Karyotaki, 2021).
Meta-analysis by Jiang et al. (2022) with 21 included RCTs found significant improvements in perinatal depression from cognitive behavioral therapy (CBT) and interpersonal therapy (IPT); IPT was more effective than CBT, and brief interpersonal therapy (IPT-B) was better than standard IPT. In a prospective randomized clinical trial of pregnant patients from diverse backgrounds with major depressive disorder, IPT-B led to significant improvement in depressive symptoms compared to enhanced usual care (Hankin et al., 2023).
Fatherhood and Mental HealthAlthough it is frequently underrecognized, misdiagnosed, and undertreated, the prevalence of paternal postpartum depression is estimated between 1.2% and 25.5% and is associated with paternal unemployment and psychological status, maternal mental illness, first pregnancy, and quality of marital relationship (Wang et al., 2021). In addition to the lack of awareness and screening for paternal mental illness, men with perinatal depression are more likely than women to present with anger, irritability, or interpersonal conflict, which can lead to misdiagnosis (Skilbeck et al., 2023).
Risks of Paternal Perinatal DepressionPaternal perinatal depression is correlated with maternal perinatal depression (Skilbeck et al., 2023). Additionally, Ashraf et al. (2023) reports that poor paternal mental health, particularly depression, at any stage of child development is associated with a broad range of negative impacts on children, including: Increased distress in infants and impaired socioemotional development Disruptive behavior, including oppositional defiant disorder (ODD), conduct disorder (CD), and attention-deficit/hyperactivity disorder (ADHD) Neurodevelopmental disorders, such as increased rates of autism spectrum disorders (ASD) Impaired speech and language development “Hostile home environments and dysfunctional family dynamics” Low income, which can further impact maternal and infant health
Benefits of Involved FathersPaternal involvement is associated with better early infant neurodevelopment, and this effect is partially mediated by a resulting reduction of parenting stress in mothers (Kim et al., 2016). Research also shows that supportive fathers can help moderate the effects of maternal adverse childhood experiences (ACEs) on prenatal depressive symptoms, although mothers with high ACE scores are still at increased risk of prenatal depressive symptoms despite high paternal support (Fields et al., 2022). A prospective study of 95 pregnant women found that involvement of the father of the baby was associated with decreased depressive symptoms and better psychological well-being (Giurgescu & Templin, 2015). A Japanese cohort study of over 18,000 children found that more active paternal involvement in childcare tasks (like changing diapers) at 6 months of age was associated with greater psychological well-being at 16 years old (Kato et al., 2023).
Treatment of Paternal Mental IllnessWhile there is limited evidence about treatment of perinatal mental health treatment of fathers, one case study by Skilbeck et al. (2023) reports significant improvement of paternal perinatal depression symptoms in a 22-year-old first-time father taking 20 mg citalopram after attending 12 weekly sessions of cognitive behavioral therapy (CBT) over 4 months, with maintenance at 3-month follow-up:

Data from Skilbeck et al. (2023) See also: Raising An Emotionally Intelligent Child
Exercise Effects on Perinatal Depression and AnxietyHigh levels of physical activity during pregnancy are associated with lower risk of prenatal depression and anxiety, as well as decreased severity of prenatal depression and anxiety symptoms, reduced stress, and increased quality of life, although pre-pregnanc
|
 More
More
 Religion & Spirituality
Religion & Spirituality Education
Education Arts and Design
Arts and Design Health
Health Fashion & Beauty
Fashion & Beauty Government & Organizations
Government & Organizations Kids & family
Kids & family Music
Music News & Politics
News & Politics Science & Medicine
Science & Medicine Society & Culture
Society & Culture Sports & Recreation
Sports & Recreation TV & Film
TV & Film Technology
Technology Philosophy
Philosophy Storytelling
Storytelling Horror and Paranomal
Horror and Paranomal True Crime
True Crime Leisure
Leisure Travel
Travel Fiction
Fiction Crypto
Crypto Marketing
Marketing History
History

.png)
 Comedy
Comedy Arts
Arts Games & Hobbies
Games & Hobbies Business
Business Motivation
Motivation